Title: The Four states of Matter
Author: Alloya Huckfield
Description: Explore the four fundamental states of matter—solids, liquids, gases, and plasma—along with their unique properties, phase transitions, and real-world examples. Discover how matter behaves under different conditions and the science behind everyday phenomena.
tags:
- solids
- states
- Matter
- AbZu
- liquids
- plasma
- gases
icon: LiAsteriskthe-four-states-of-matter
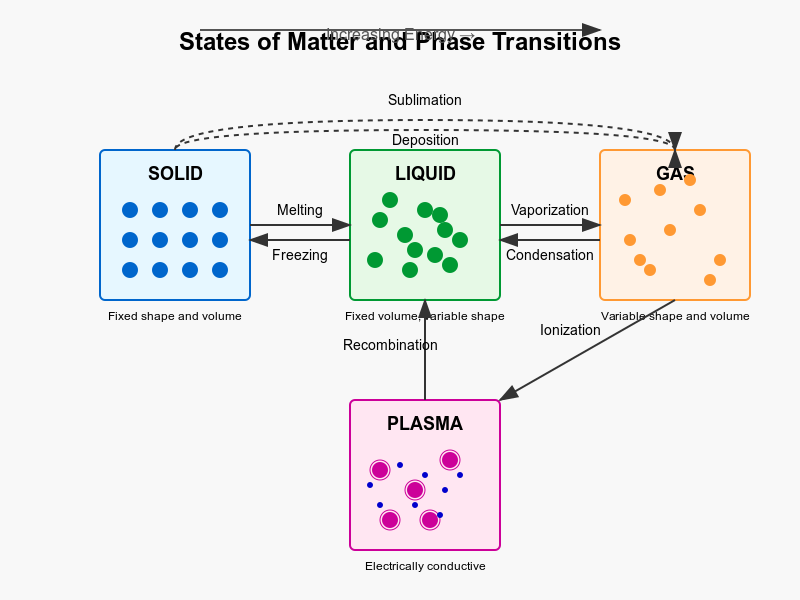
Matter exists in several distinct states characterized by unique physical properties and molecular behaviours. While we commonly encounter three states in everyday life—solids, liquids, and gases—there is a fourth fundamental state called plasma. Let's examine each of these states and the transitions between them.
Solid State
Solids maintain a fixed volume and shape regardless of their container. This stability comes from the strong intermolecular forces that hold particles in fixed positions relative to each other. At the molecular level, particles in solids:
- Vibrate in place around fixed positions
- Maintain regular, ordered arrangements (in crystalline solids)
- Have the highest density among common states
- Resist compression and flow
Examples of solids include ice, rocks, metals, and wood. While particles in solids have minimal freedom of movement, they still possess thermal energy and vibrate more intensely as temperature increases.
In crystalline solids like diamond or table salt, particles arrange in repeating three-dimensional patterns called crystal lattices. These structures give crystals their characteristic shapes and planes of cleavage. Amorphous solids like glass or plastic lack this long-range order, with particles arranged more randomly despite strong intermolecular bonds.
Liquid State
Liquids maintain a fixed volume but conform to the shape of their container. Particles in liquids:
- Move freely past one another
- Maintain close proximity with moderate intermolecular attraction
- Flow readily while remaining relatively incompressible
- Form a distinct surface at their upper boundary
Examples include water, oil, and mercury. The particles in liquids have enough energy to break from fixed positions but not enough to separate completely from neighbouring particles. This balance allows liquids to flow while maintaining cohesion.
Surface tension—the property that allows insects to walk on water or drops to form spherical shapes—results from the inward pull of these intermolecular forces at the liquid's surface. Viscosity, a liquid's resistance to flow, varies widely between substances depending on molecular size, shape, and attraction forces.
Gas State
Gases expand to fill any container completely, with both variable volume and shape. Particles in gases:
- Move rapidly and randomly
- Have minimal intermolecular attractions
- Maintain large average distances between particles
- Undergo frequent elastic collisions with each other and container walls
Common gases include oxygen, nitrogen, and carbon dioxide. Gas particles possess significant kinetic energy, moving at hundreds of meters per second at room temperature. This energy overcomes most intermolecular attractions, allowing gases to expand indefinitely unless contained.
The kinetic molecular theory explains gas behaviour through these rapid molecular motions. Gas pressure results from countless molecular collisions with container walls. As temperature increases, molecular speed increases, resulting in more frequent and forceful collisions that produce higher pressure.
Plasma State
Plasma, often called the fourth state of matter, occurs when gas is heated to extremely high temperatures or subjected to strong electromagnetic fields. In this state:
- Electrons separate from atoms, creating a mixture of positively charged ions and free electrons
- The material becomes electrically conductive
- Particles respond strongly to electromagnetic forces
- Complex collective behaviors emerge that aren't seen in other states
Though less familiar in everyday life, plasma is actually the most abundant state of matter in the universe. Stars, including our sun, consist primarily of plasma. On Earth, we encounter plasma in lightning, flames, auroras, and fluorescent lights.
Plasma exhibits unique properties including the ability to generate and be shaped by magnetic fields. Unlike neutral gases, plasmas can conduct electricity and respond to electromagnetic fields as a collective. This enables applications ranging from plasma televisions to fusion energy research.
Phase Transitions
Transitions between states occur when energy is added or removed from a substance, typically in the form of heat:
- Melting: Solid to liquid (energy absorbed)
- Freezing: Liquid to solid (energy released)
- Vaporization: Liquid to gas (energy absorbed)
- Condensation: Gas to liquid (energy released)
- Sublimation: Solid directly to gas (energy absorbed)
- Deposition: Gas directly to solid (energy released)
- Ionization: Gas to plasma (energy absorbed)
- Recombination: Plasma to gas (energy released)
During phase transitions, temperature remains constant while the material absorbs or releases energy as "latent heat." This energy goes into breaking intermolecular bonds rather than increasing molecular motion. For example, ice at exactly 0°C absorbs significant heat without temperature change as it melts into water at 0°C.
The conditions required for phase transitions depend on both temperature and pressure. These relationships are mapped in phase diagrams, which show the regions where each state is stable and the boundaries where transitions occur. At the triple point, a unique combination of temperature and pressure allows all three common states to coexist in equilibrium.
Beyond the Four States
While the four states described represent the fundamental classifications of matter, scientists recognize additional states under extreme conditions:
- Bose-Einstein condensates: Formed near absolute zero, where particles merge into a single quantum state
- Supercritical fluids: Materials above their critical point that have properties of both liquids and gases
- Quark-gluon plasma: An ultra-high energy state where even protons and neutrons break apart
- Neutron-degenerate matter: Found in neutron stars, where electron pressure cannot resist gravitational collapse
These exotic states reveal that our understanding of matter continues to evolve as we explore more extreme conditions of temperature, pressure, and density.
The study of matter's states illuminates fundamental principles of physics while explaining countless everyday phenomena—from why ice floats on water to how pressure cookers work to what powers the sun. This understanding forms the foundation for everything from materials science to astrophysics.